LUMOS DIAGNOSTICS HOLDINGS ORD

Lumos Diagnostics Achieves BARDA Paediatric Study Milestone, Secures US$720k Payment
Lumos Diagnostics hits BARDA Milestone 6, secures US$720k non-dilutive payment as FebriDx paediatric study advances toward FDA submission and wider US TAM.
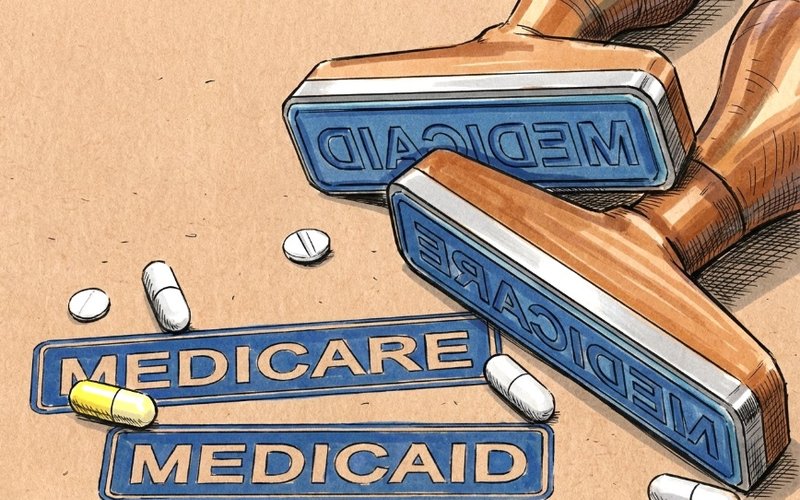
Lumos Diagnostics Secures Full US Medicare Reimbursement Recognition for FebriDx Test
Lumos Diagnostics (ASX: LDX) has achieved complete US Medicare reimbursement recognition for its FebriDx point-of-care diagnostic test, securing coverage from all seven Medicare Administrative Contractors.
Lumos Diagnostics secures US government funding to develop groundbreaking FebriDx test
Lumos Diagnostics (ASX: LDX) has received significant US financial backing for its development of unique point-of-care diagnostic test technology. Lumos has been awarded approximately $4.3 million by a division of the US government’s health division to support a planned study and regulatory submission for the company’s FebriDx bacterial/non-bacterial test. FebriDx is designed to assess whether […]
Lumos Diagnostics extends distribution of FebriDx point-of-care test into Belgian market
Lumos Diagnostics (ASX: LDX) has extended a distribution agreement for its FebriDx rapid point-of-care test to include the Belgian market of US healthcare products supplier Henry Schein Medical. FebriDx is designed to identify viral versus bacterial acute respiratory infections within 10 minutes from a fingerstick blood sample and can be used to help accurately manage […]