AVITA MEDICAL CDI
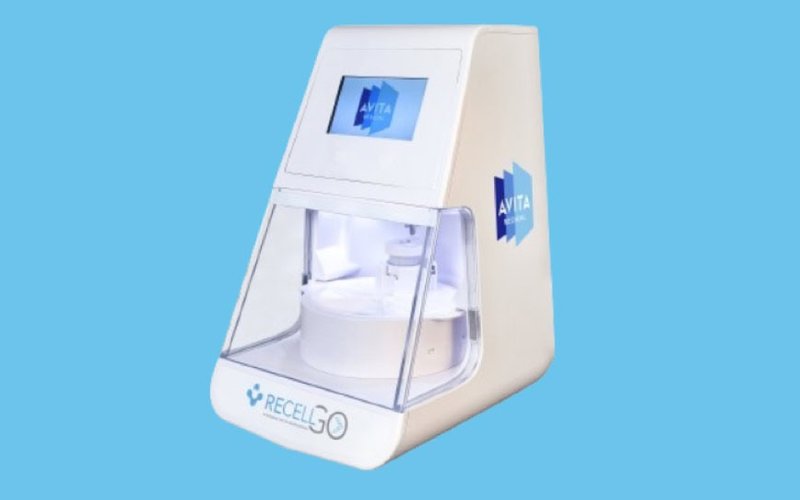
AVITA Medical to launch RECELL GO system in US burn centres following FDA approval
Wound care management specialist AVITA Medical (ASX: AVH) has obtained US Food and Drug Administration (FDA) approval for its RECELL GO skin restoration system. RECELL GO is a cell-harvesting device that utilises the regenerative properties of a patient’s own skin to treat thermal burn wounds and full-thickness skin defects. Chief executive officer Jim Corbett said […]

What are the ASX stocks vulnerable to a ‘short squeeze’?
Could the ASX become subject to the same trading shenanigans in the US which put a rocket up the share price of struggling video outlet operator GameStop and then the silver market. It’s certainly food for short … er, thought. This month shares in online travel agent Webjet (ASX: WEB) have climbed around 10% as […]
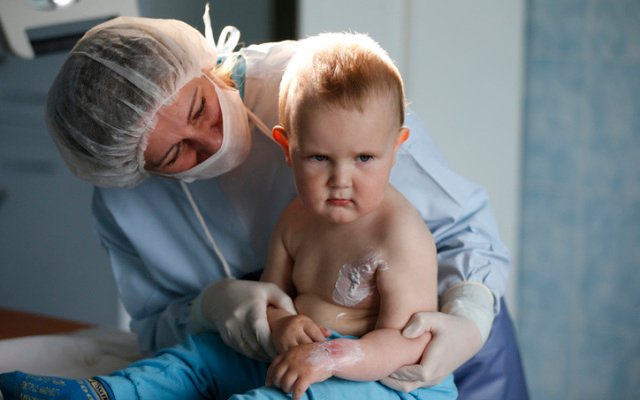
Avita Medical enrols first patients in studies evaluating RECELL system
Regenerative medicine company Avita Medical (ASX: AVH) has commenced what it calls a “pivotal trial” for the treatment of paediatric scald injuries, seeking to demonstrate that its RECELL treatment can safely and effectively increase the incidence of healing when compared to a standard wound dressing. The new study is Avita’s second in as many days after […]
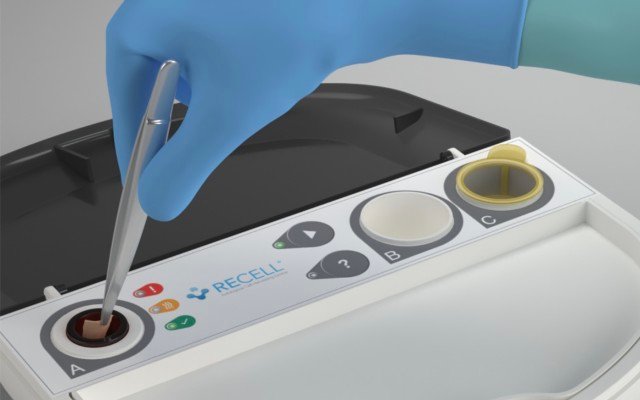
AVITA Medical publishes favourable study results for its RECELL System
Regenerative medicine company AVITA Medical (ASX: AVH) has published “favourable” results from an open-label feasibility study of its RECELL Autologous Cell Harvesting Device for the treatment of diabetic foot ulcers (DFUs). AVITA Medical has positioned itself as an innovative technology developer, intending to address unmet medical needs in burns, chronic wounds, and aesthetics indications. AVITA’s device […]